

Raman spectroscopic analysis of functions of human Galectin-1
Resonance Raman spectroscopy
When we use Raman spectroscopy biochemical studies, the data analysis is often complicated because many bands of the Raman-active modes are observed. The number of the band is huge because biomolecules consist of thousands of atoms in many cases. We may use the resonance effect in Raman spectroscopy by tuning the excitation wavelength to an electronic transition of chlomophore; finite number of the Raman bands of the chromophore is selectively enhanced and observed in the spectrum. We use 229-nm excitation beam that resonates the electronic absorption of the aromatic groups, in particular tryptophane and histidine, in proteins.
Galectin-1
Human galectin-1 (Gal-1) is a lectin and is known to have affinity to ΐ-galactoside. Gal-1 exists in homodimer under the physiological functions, and possesses two pockets to bind the carbohydrate in the dimer. The Gal-1 dimer binds the ligands at each pocket, thereby bridging two molecules. Intercellular bridging of the carbohydrate moieties on different cells assists cell adhesion, while the intracellular bridging assembles membrane proteins in the cell. Owing to these functions, Gal-1 plays important roles in cell physiology.
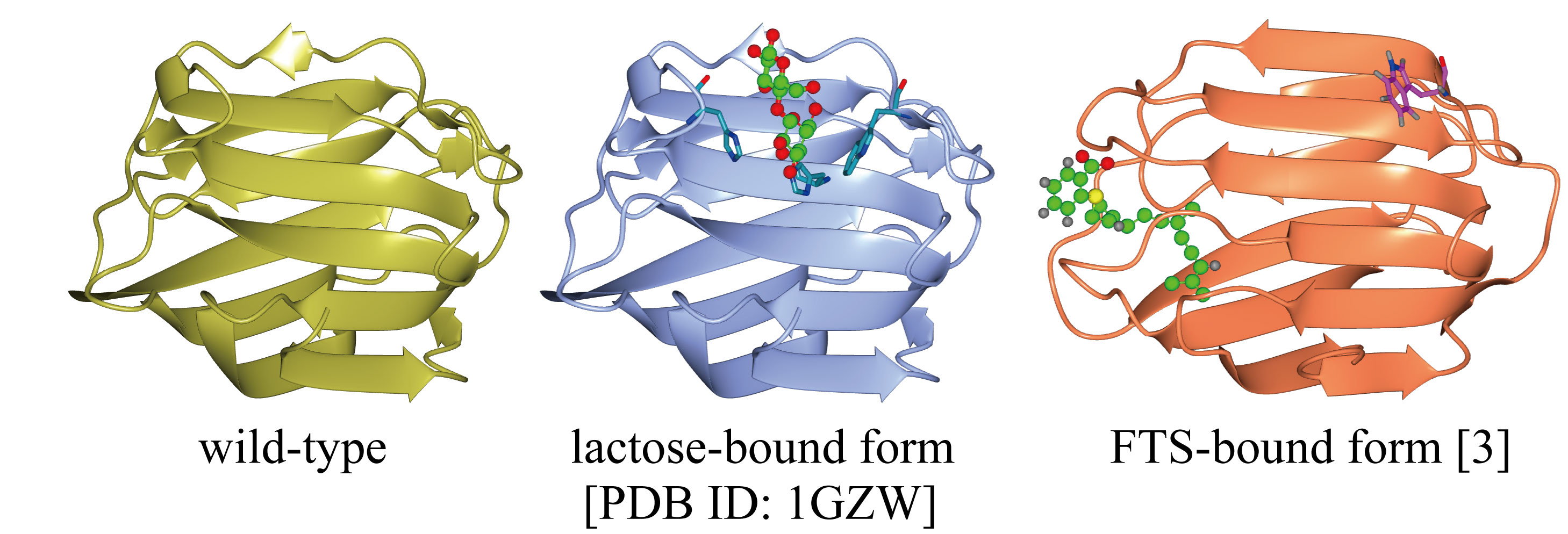
Affinity to lactose: origin of pH-dependence
Gal-1 involves two histidines, His44 and His52. These residues directly interact with lactose (Galΐ1-4Glc). We found that the lactose binding constant is dependent on pH at the acidic pH. The dependence is due to structural change of the binding pocket upon the protonation of His52 (pKa 6.2) and that of His44 (pKa 5.7) [1].
It is reported that the amount of Gal-1 bound to agalose gel carrying the lactose moieties decreases at pH >7.5, whereas we found that the lactose binding constant was unchanged at pH 7-9.5. This dependence was explained by taking account of the deprotonation of Cys (pKa was determined from Raman spectrum to be 8.5), which is at protein surface, and resultant Coulombic repulsion [2].
Affinity to the farnesyl group: occurrence of self-clustering
It is considered that Gal-1 serves not only as the lectin but also as a receptor of the farnesyl group. The farnesyl group consists of three isoprene unit and posttranslationally attached to the C-terminal Cys of H-Ras. H-Ras forms a cluster and plays a crucial role in cell growth. Importance of the farnesyl bound Gal-1 has been pointed out in the clustering of H-Ras. We found that Gal-1 in the farnesyl-bound form generated self-clusters by adding farnesyl thiosalicylic acid (FTS), and studied the structure of farnesyl-bound Gal-1 using UV-Raman and fluorescence spectroscopy [3]. The self-clustering of the farnesyl-bound Gal-1 may explain the clustering of H-Ras (farnesyl-dependent model).
[1] H.Hiramatsu, K.Takeuchi, H.Takeuchi, Biochemistry 2013, 52, 2371-2380.
[2] H.Hiramatsu, K.Takeuchi, K.Fukuda, T.Nishino, Chemical Physics 2013, 419, 113-117.
[3] K.Yamaguchi, Y.Niwa, T.Nakabayashi, H.Hiramatsu, Scientific Reports, 2016, 6, 32999.