

Infrared electroabsorption spectroscopy
The development of novel spectroscopic techniques is quite important in natural sciences because it serves as a new tool to elucidate mechanisms and rules underlying natural phenomena. Fundamental properties of molecules like chemical bonding and reactivity are influenced profoundly by electrostatic interactions, and it is of importance to elucidate these interactions by observing the responses of molecules to an applied external electric field. Such kinds of experiments (Stark spectroscopy) have been performed extensively using UV-visible absorption and fluorescence spectra, whereas there were only a few vibrational spectroscopic studies.
We have developed infrared electroabsorption spectroscopy by combining dispersive IR spectrometer and the AC coupled amplifier system, and succeeded in detecting the ΔA signal of 1,2-dichloroethane as small as 10-7 in 2001 [1]. This experiment is a novel technique that provides information not only on the electronic properties of vibrationally excited states (as has been shown in the UV-visible region) but also on the electric field effect on the molecular structure [2]. Using this apparatus, we have succeeded in detecting the IR electroabsorption signal of organic liquid molecules at room temperature.
The orientational polarization gives rise to the ΔA signal and brings the information about the permanent dipole moment μP and η (the angle between μP and the vibrational permanent dipole moment). Association structures in solution have been detected so far with N-methylacetamide [3], liquid crystal (5CB) [4], p-nitroaniline/acetonitrile [5], and 1-hydroxyacetone [6]. The orientational polarization signal originates from an anisotropic distribution of the polar molecules induced by the external electric field. The orientational polarization signal is separable from other signals because it disappears from the ΔA spectrum when the angle (χ) between the electric field vector of the IR light and the applied electric field vector is set to 54.7°.
Effects on ketose-aldose equilibrium of saccharide[6,7]
The electric field affects chemical equilibrium, and the change in the number of molecules is detected as a change in the ΔA spectrum. This effect is explained regarding additional energy due to an interaction between the permanent dipole moment and the applied electric field.
Monosaccharide exists in an equilibrium of the cyclic and acyclic forms. The acyclic form exists in an equilibrium of ketose and aldose. We have studied the electric field effects on the ketose-aldose equilibrium of the acyclic monosaccharide. 1-hydroxyacetone (1-HA) [6] and 1,3-dihydroxyacetone (1,3-DHA) [7] were chosen as the acyclic aldose. The electric field on the aldose induced the orientational polarization and the change in the fraction of tautomer. We could explain the changes in the IR spectrum by considering the electric field effect on the free energy differences, μP and η, and the vibrational transition moment) of each tautomer, and the electric field intensity. Three-state equilibrium was concluded for 1-HA with the 1-HA dimer, 1-HA monomer, and 1- or 2-propene-1,2-diol. The five-state equilibrium of the ketose, Z-enediol, two E-enediol structures, and the aldose explained the result of 1,3-DHA.
The IR electroabsorption spectroscopy brings the information of molecular structure (μP,η) that the IR absorption spectroscopy does not, and thus provides the new tool for the structural chemistry.
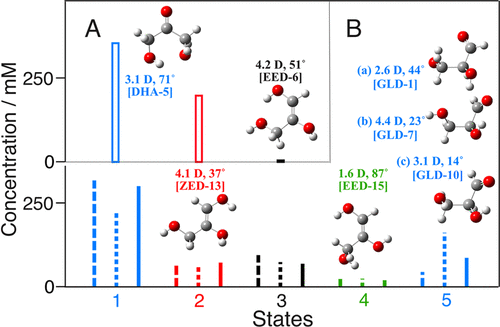
[Reprinted with permission from [7] J. Phys. Chem. B, 2019, 123, 10633-10671. Copyright 2020 American Chemical Society.]
[1] H.Hiramatsu, C.Kato, H.Hamaguchi, Chem. Phys. Lett. 2001, 347, 403-409.
[2] H.Hiramatsu, H.Hamaguchi, Appl. Spectrosc. 2004, 58, 355-366.
[3] H.Hiramatsu, H.Hamaguchi, Chem. Phys. Lett. 2002, 361, 457-464.
[4] Y.-K.Min, H.Hiramatsu, H.Hamaguchi, Chem. Lett. 2002, 68-69;
[5] S.Shigeto, H.Hiramatsu, H.Hamaguchi, J. Phys. Chem. A 2006, 110, 3738-3743.
[6] H.Hiramatsu, Chem. Phys. Lett. 2019, 714, 18-23.
[7] S.H.Chen, H.Hiramatsu, J. Phys. Chem. B, 2019, 123, 10663-10671.