

Nitrosylated galectin-1
Galectin-1 (Gal-1) is one of the mammalian lectins. Gal-1 has an affinity to ā└-galactoside when its six Cys residues exist in the S-H form. Gal-1 serves as the lectin under reductive conditions.
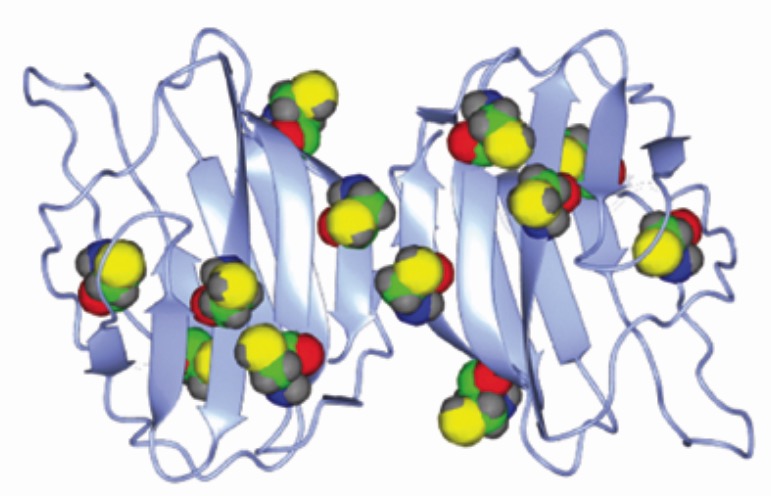
Fig. Structure of Gal-1 dimer. The Cys residues are highlighted. Reproduced from Ref.1 with permission from the Royal Society of Chemistry.
Formation of the disulfide bridges under oxidative conditions largely alters the main chain structure of Gal-1 and consequently decreases the lectin activity. Cys->Ser mutants of Gal-1 retain the affinity even under the oxidative conditions.
We hit on an idea that nitrosylation of the Cys side chains of Gal-1 is interesting. It will have the affinity regardless of the conditions by analogy with the Cys->Ser mutants, and irradiation of the light will cleave the S-NO bond and restore the protein structure. Then, under the oxidative conditions, Gal-1 will undergo the photoinduced transformation, which can trigger the lose of affinity of Gal-1. That is, we tried to develop a protein of which the property is changed with the photoirradiation.
By establishing a protocol of efficient and tricky method of the nitrosylation of the Cys side chains using metallothionein, we succeeded in preparing the nitrosylated Gal-1 (SNO-Gal1) and attained the photocontrol of the lectin activity with an irradiation of 355-nm light. Hence, we achieved the photoinduced change in the affinity of protein.
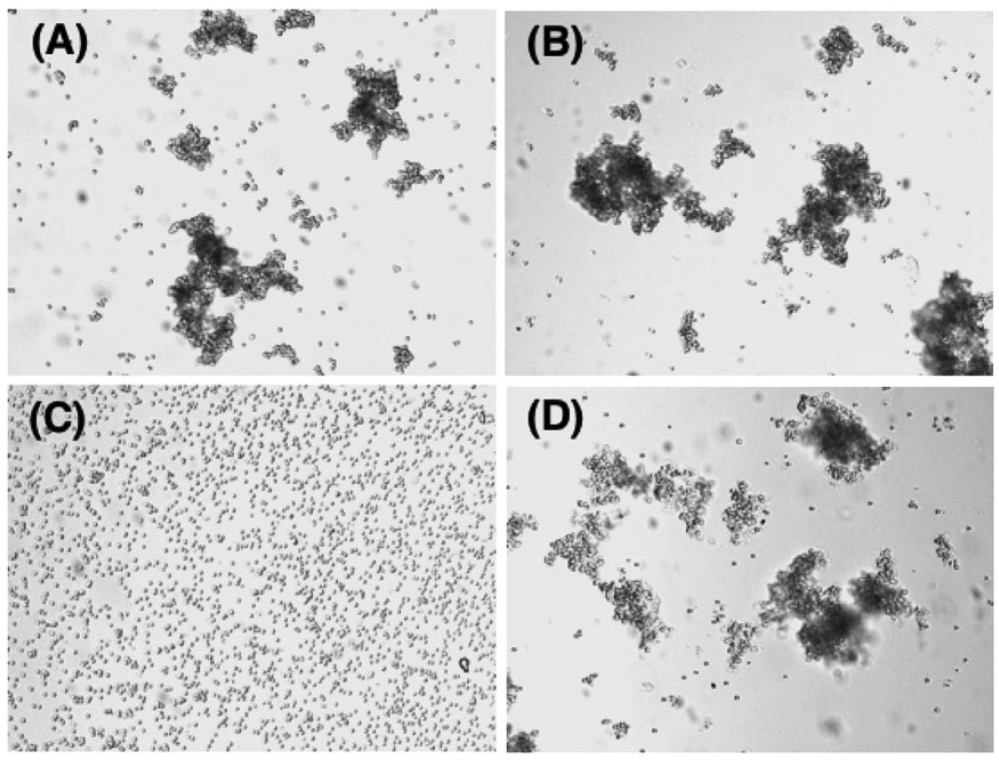
Fig. Effect of photoirradiation on aggregates of rabbit erythrocytes formed by SNO-hGal-1 (A-C) or WT-hGal-1 (D). (A) SNO-Gal-1, before photoirradiation, (B) SNO-Gal-1, without photoirradiation, (C) SNO-Gal-1, with photoirradiation, and (D) native Gal-1, with photoirradiation. Reproduced from Ref.1 with permission from the Royal Society of Chemistry.
[1] T.Kurota, I.Sato, K.Kuroi, H.Hiramatsu, T.Nakabayashi, Chem. Commun, 2017, 53, 10014-10017.
[2] K.Kuroi, M.Kamijoh, M.Ueki, H.Hiramatsu, T.Nakabayashi, Phys. Chem. Chem. Phys., accepted (2020).